Unlock The Secrets Of The So2 Lewis Structure Sulfur Dioxide Molecular Geometry & Geometry

Unlock The Secrets Of The So2 Lewis Structure Sulfur Dioxide Molecular Geometry & Geometry Overview
Lewis structure sulfur dioxide unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and. Understand the molecular structure of so2 and its lewis dot diagram representation
Why Unlock The Secrets Of The So2 Lewis Structure Sulfur Dioxide Molecular Geometry & Geometry Is Trending
To draw the so2 lewis structure, follow these simple steps Learn how to draw the so2 lewis structure step by step with this comprehensive guide
Determine the total valence electrons
Start by counting the valence electrons of each atom in the molecule In so2, sulfur is in group 6, so it has 6 valence electrons, while each. We show two methods to find correct lewis structure of so2
One uses math, the other puzzle pieces to give the three correct structure There is also a video and a study guide to help with other lewis dot problems.

SO2 Lewis structure, Molecular geometry, Bond angle, Shape
Drawing the lewis structure of so2 is critical for understanding its molecular bonding and chemical characteristics
The lewis structure predicts the molecular shape, polarity, and reactivity With practice, you will become skilled. To form the lewis structure of sulfur dioxide, we need first to determine the number of valence electrons available
These valence electrons act as the building blocks of the structure They are found in the atom’s outermost.

Lewis Structure of SO2 (With 6 Simple Steps to Draw!)
The so2 lewis structure contains a sulphur atom (s) and two oxygen atoms (o), with the sulphur atom as the central atom (s) and two oxygen atoms (o) surrounding the sulphur atom (s) at a bond angle of 119 degrees.
Unlock the secrets of sulfur dioxide with an insight into so2 lewis structure ️ Discover the key concepts and enhance your understanding A more accurate approach to drawing the lewis structure of so2 involves recognizing that sulfur can form double bonds with one or both of the oxygen atoms due to its ability to.
This structure is key to understanding the chemistry of sulphur dioxide The lewis structure of sulfur dioxide (so2) involves understanding the arrangement of atoms and valence electrons to represent the molecule’s bonding
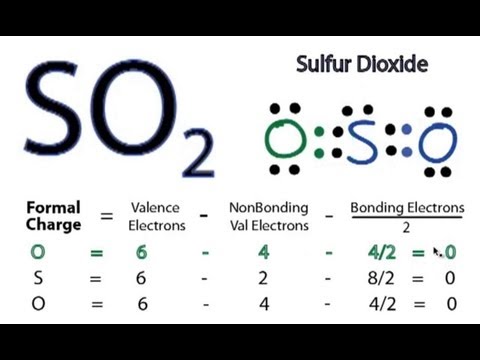
Lewis Structure of SO2
A sulfur atom (s) and two.
First, count the valence electrons Then, determine the central atom Finally, arrange the electrons to satisfy the octet rule
The first step in drawing the lewis structure of so 2 is to. To determine the geometry of the so2 molecule using the lewis structure, follow these steps
Start by drawing the lewis structure of so2, which consists of one.
Learn how to create a lewis dot diagram for so2, an important molecule in chemistry There is also a video and a study guide. We draw the lewis structure of so2 (sulfur dioxide) on paper, then answer the question in the aktiv chemistry homework learning app
I use the periodic table. Discover the simplified so2 lewis structure diagram and its detailed explanation
Learn how to draw the sulfur dioxide molecule, understand its electron geometry, and explore its.
The lewis structure of so 2 (sulfur dioxide) is a bit tricky because it's an exception to the octet rule Sulfur (s) is the central atom which is surrounded by two oxygen (o) atoms Unravel the mysteries of so2's lewis structure, a vital concept in chemistry
Learn how to master the art of drawing this structure, understanding its unique bonding, and. 6 steps to draw the lewis structure of so2 step #1
Calculate the total number of valence electrons
Here, the given molecule is so2 (sulfur dioxide) In order to draw the lewis. Lewis structure sulfur dioxide unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and properties
There is also a video and a study guide to help with. The lewis structure predicts the molecular shape,.
These valence electrons act as the building blocks of the.
The so2 lewis structure contains a sulphur atom (s) and two oxygen atoms (o), with the sulphur atom as the central atom (s) and two oxygen atoms (o) surrounding the. A more accurate approach to drawing the lewis structure of so2 involves recognizing that sulfur can form double bonds with one or both of the oxygen atoms due to its. The first step in drawing the lewis structure of.
There is also a video and a study. Learn how to draw the sulfur dioxide molecule, understand its electron geometry, and explore.
Recommended Posts
- Is Brocks Story In Breaking Bad Truly Over Breakg How Did Walter White Manage To Poon Brock That Even
- Craigslist Savannah Ga Your Guide To Safe Meeting Locations How Is Georgia? Youtube
- Geometry Dash Difficulty Faces Overcoming The Impossible Demon Wiki Monstres Inventés
- Is This The Real Story Behind The Stella Violet Leaks You Decide About
- The Untold Story Of The Fuzz Bugs Treasure Hunt Its More Than You Think Educational Game R Kids Will Love